What Is Stress Corrosion Cracking
Stress corrosion cracking (SCC) is the growth of crack formation in a corrosive environment. It can lead to unexpected sudden failure of normally ductile metal alloys subjected to a tensile stress, especially at elevated temperature. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when exposed to a small number of chemical environments. The chemical environment that causes SCC for a given alloy is often one which is only mildly corrosive to the metal. Hence, metal parts with severe SCC can appear bright and shiny, while being filled with microscopic cracks. This factor makes it common for SCC to go undetected prior to failure. SCC often progresses rapidly, and is more common among alloys than pure metals. The specific environment is of crucial importance, and only very small concentrations of certain highly active chemicals are needed to produce catastrophic cracking, often leading to devastating and unexpected failure.[1]
The stresses can be the result of the crevice loads due to stress concentration, or can be caused by the type of assembly or residual stresses from fabrication (e.g. cold working); the residual stresses can be relieved by annealing or other surface treatments.
- 6Prevention
Metals attacked[edit]
Stress corrosion cracking (SCC) of Mg alloys is intergranular (IGSCC) or transgranular (TGSCC). A continuous or nearly continuous second phase, typically along grain boundaries, causes IGSCC by micro-galvanic corrosion of the adjacent Mg matrix. Nov 11, 2018 This explains the stress corrosion and the conditions. Three different mechanisms involved in stress corrosion. Explains caustic embrittlement, season cracking, hydrogen embrittlement.
Certain austeniticstainless steels and aluminiumalloys crack in the presence of chlorides, mild steel cracks in the presence of alkali (boiler cracking) and nitrates, copper alloys crack in ammoniacal solutions (season cracking). This limits the usefulness of austenitic stainless steel for containing water with higher than few ppm content of chlorides at temperatures above 50 °C. Also of concern is the fact that high-tensile structural steels have been known to crack in an unexpectedly brittle manner in a whole variety of aqueous environments, especially when chlorides are present. With the possible exception of the latter, which is a special example of hydrogen cracking, all the others display the phenomenon of subcritical crack growth, i.e. small surface flaws propagate (usually smoothly) under conditions where fracture mechanics predicts that failure should not occur. That is, in the presence of a corrodent, cracks develop and propagate well below KIc. In fact, the subcritical value of the stress intensity, designated as KIscc, may be less than 1% of KIc, as the following table shows:
| Alloy | KIc MN/m3/2 | SCC environment | KIscc MN/m3/2 |
|---|---|---|---|
| 13Cr steel | 60 | 3% NaCl | 12 |
| 18Cr-8Ni | 200 | 42% MgCl2 | 10 |
| Cu-30Zn | 200 | NH4OH, pH7 | 1 |
| Al-3Mg-7Zn | 25 | Aqueous halides | 5 |
| Ti-6Al-1V | 60 | 0.6M KCl | 20 |
Polymers attacked[edit]
A similar process (environmental stress cracking) occurs in polymers, when products are exposed to specific solvents or aggressive chemicals such as acids and alkalis. As with metals, attack is confined to specific polymers and particular chemicals. Thus polycarbonate is sensitive to attack by alkalis, but not by acids. On the other hand, polyesters are readily degraded by acids, and SCC is a likely failure mechanism. Polymers are susceptible to environmental stress cracking where attacking agents do not necessarily degrade the materials chemically.Nylon is sensitive to degradation by acids, a process known as hydrolysis, and nylon mouldings will crack when attacked by strong acids.
Stress corrosion cracking (SCC) is a type of environmentally-assisted cracking (EAC), or the formation of cracks caused by various factors combined with the environment surrounding the pipeline. SCC occurs as a result of a combination between corrosion and tensile stress. Corrosion is. Polythionic Acid Stress Corrosion Cracking (PASCC) is an affliction of many refineries processing sulfur containing feedstocks, and since that is the norm these days, most refiners reduce their susceptibility to PASCC by selecting resistant alloys orby neutralizing exposed surfaces during shutdowns. Chloride stress corrosion cracking (CLSCC) is one the most common reasons why austenitic stainless steel pipework and vessels deteriorate in the chemical processing and petrochemical industries. Deterioration by CLSCC can lead to failures that have the potential to release stored energy and/or hazardous substances. Stress-corrosion cracking (SCC) is a term used to describe service failures in engineering materials that occur by slow, environmentally induced crack propagation. The observed crack propaga tion is the result of the combined and synergistic interaction of mechanical stress and corrosion re actions. Before SCC can be discussed in detail, we.
For example, the fracture surface of a fuel connector showed the progressive growth of the crack from acid attack (Ch) to the final cusp (C) of polymer. In this case the failure was caused by hydrolysis of the polymer by contact with sulfuric acid leaking from a car battery. The degradation reaction is the reverse of the synthesis reaction of the polymer:
In short, you can open an animated GIF image with the Photos app to view the animated GIF. Photos, the replacement for the classic, supports GIFs. You can use these apps to open and view animated GIFs only. Windows 10 download free.
Cracks can be formed in many different elastomers by ozone attack, another form of SCC in polymers. Tiny traces of the gas in the air will attack double bonds in rubber chains, with natural rubber, styrene-butadiene rubber, and nitrile butadiene rubber being most sensitive to degradation. Ozone cracks form in products under tension, but the critical strain is very small. The cracks are always oriented at right angles to the strain axis, so will form around the circumference in a rubber tube bent over. Such cracks are very dangerous when they occur in fuel pipes because the cracks will grow from the outside exposed surfaces into the bore of the pipe, so fuel leakage and fire may follow. The problem of ozone cracking can be prevented by adding anti-ozonants to the rubber before vulcanization. Ozone cracks were commonly seen in automobile tire sidewalls, but are now seen rarely thanks to the use of these additives. On the other hand, the problem does recur in unprotected products such as rubber tubing and seals.
Ceramics attacked[edit]
This effect is significantly less common in ceramics which are typically more resilient to chemical attack. Although phase changes are common in ceramics under stress these usually result in toughening rather than failure (see Zirconium dioxide). Recent studies have shown that the same driving force for this toughening mechanism can also enhance oxidation of reduced cerium oxide, resulting in slow crack growth and spontaneous failure of dense ceramic bodies.[2]
Glass attacked[edit]
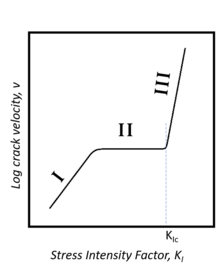
Given that most glasses contain a substantial silica phase, the introduction of water can chemically weaken the bonds preventing subcritical crack propagation. Indeed, the silicon oxygen bonds present at the tip of a crack are strained, and thus more susceptible to chemical attack. In the instance of chemical attack by water, silicon-oxygen bonds bridging the crack are separated into non-connected silicon hydroxide groups. The addition of external stress will serve to further weaken these bonds. Subcritical crack propagation in glasses falls into three regions. In region I, the velocity of crack propagation increases with ambient humidity due to stress-enhanced chemical reaction between the glass and water. In region II, crack propagation velocity is diffusion controlled and dependent on the rate at which chemical reactants can be transported to the tip of the crack. In region III, crack propagation is independent of its environment, having reached a critical stress intensity. Chemicals other than water, like ammonia, can induce subcritical crack propagation in silica glass, but they must have an electron donor site and a proton donor site.[3]
Crack growth[edit]
The subcritical nature of propagation may be attributed to the chemical energy released as the crack propagates. That is,
Get it done right with Avery Design and Print and a variety of other templates and software at Avery.com. Use Microsoft Word templates and Adobe templates to design and print the easy way. Sep 13, 2017 Indien Server for Interface 80 Label Template 5160.Easily down load free almost eight. 5″ times 11″ sticker templates to get laser and inkjet creating. We offer a whole collection of catchphrase templates which include popular COMPACT DISK Blank Ingredients label.  Printable Tags. Shop All Tags. Templates for Tags. Name Tags & Badges. Blank Name Tags & Badges. Custom Printed Name Tags & Badges. Name Tags & Badges. Find a Template Avery Design & Print View All Templates. Cobrowse with Avery. Shop our brands. Select a Country. United States - English.
Printable Tags. Shop All Tags. Templates for Tags. Name Tags & Badges. Blank Name Tags & Badges. Custom Printed Name Tags & Badges. Name Tags & Badges. Find a Template Avery Design & Print View All Templates. Cobrowse with Avery. Shop our brands. Select a Country. United States - English.
- elastic energy released + chemical energy = surface energy + deformation energy
The crack initiates at KIscc and thereafter propagates at a rate governed by the slowest process, which most of the time is the rate at which corrosive ions can diffuse to the crack tip. As the crack advances so K rises (because crack length appears in the calculation of stress intensity). Finally it reaches KIc, whereupon fast fracture ensues and the component fails. One of the practical difficulties with SCC is its unexpected nature. Stainless steels, for example, are employed because under most conditions they are 'passive', i.e. effectively inert. Very often one finds a single crack has propagated while the rest of the metal surface stays apparently unaffected. The crack propagates perpendicular to the applied stress.
Prevention[edit]
SCC is the result of a combination of three factors – a susceptible material, exposure to a corrosive environment, and tensile stresses above a threshold. If any one of these factors are eliminated, SCC initiation becomes impossible. However, there are a number of approaches that can be used to prevent or at least delay the onset of SCC. In an ideal world a stress corrosion cracking control strategy will start operating at the design stage, and will focus on the selection of material, the limitation of stress and the control of the environment. The skill of the engineer then lies in selecting the strategy that delivers the required performance at minimum cost. Part of the performance requirements relate to the acceptability of failure. The primary containment pressure vessel in a nuclear reactor obviously requires a very low risk of failure. For the pressed brass decorative trim on a light switch, the occasional stress corrosion crack is not going to be a serious problem, although frequent failures would have an undesirable impact on product returns and the manufacturer's image. The conventional approach to controlling the problem has been to develop new alloys that are more resistant to SCC. This is a costly proposition and can require a massive time investment to achieve only marginal success.
Selection and control of material[edit]
The first line of defence in controlling stress corrosion cracking is to be aware of the possibility at the design and construction stages. By choosing a material that is not susceptible to SCC in the service environment, and by processing and fabricating it correctly, subsequent SCC problems can be avoided. Unfortunately, it is not always quite that simple. Some environments, such as high temperature water, are very aggressive, and will cause SCC of most materials. Mechanical requirements, such as a high yield strength, can be very difficult to reconcile with SCC resistance (especially where hydrogen embrittlement is involved).
Control of stress[edit]
As one of the requirements for stress corrosion cracking is the presence of stress in the components, one method of control is to eliminate that stress, or at least reduce it below the threshold stress for SCC. This is not usually feasible for working stresses (the stress that the component is intended to support), but it may be possible where the stress causing cracking is a residual stress introduced during welding or forming.Residual stresses can be relieved by stress-relief annealing, and this is widely used for carbon steels. These have the advantage of a relatively high threshold stress for most environments, consequently it is relatively easy to reduce the residual stresses to a low enough level. In contrast austenitic stainless steels have a very low threshold stress for chloride SCC. This, combined with the high annealing temperatures that are necessary to avoid other problems, such as sensitization and sigma phase embrittlement, means that stress relief is rarely successful as a method of controlling SCC for this system.For large structures, for which full stress-relief annealing is difficult or impossible, partial stress relief around welds and other critical areas may be of value. However, this must be done in a controlled way to avoid creating new regions of high residual stress, and expert advice is advisable if this approach is adopted.Stresses can also be relieved mechanically. For example, hydrostatic testing beyond yield will tend to ‘even-out’ the stresses and thereby reduce the peak residual stress. Similarly laser peening, shot-peening, or grit-blasting tend to introduce a surface compressive stress, and are beneficial for the control of SCC. The uniformity with which these processes are applied is important. If, for example, only the weld region is shot-peened, damaging tensile stresses may be created at the border of the peened area. The compressive residual stresses imparted by laser peening are precisely controlled both in location and intensity, and can be applied to mitigate sharp transitions into tensile regions. Laser peening imparts deep compressive residual stresses on the order of 10 to 20 times deeper than conventional shot peening making it significantly more beneficial at preventing SCC.[4] Laser peening is widely used in the aerospace and power generation industries in gas fired turbine engines.[5]
Control of environment[edit]
The most direct way of controlling SCC through control of the environment is to remove or replace the component of the environment that is responsible for the problem, though this is not usually feasible. Where the species responsible for cracking are required components of the environment, environmental control options consist of adding inhibitors, modifying the electrode potential of the metal, or isolating the metal from the environment with coatings.
For example, chloride stress corrosion cracking of austenitic stainless steel has been experienced in hot-water jacketed pipes carrying molten chocolate in the food industry. It is difficult to control the temperature, while changing pipe material or eliminating residual stresses associated with welding and forming the pipework is costly and incurs plant downtime. However, this is a rare case where environment may be modified: an ion exchange process may be used to remove chlorides from the heating water.
Testing of susceptible materials[edit]
One of the primary methods used to detect and remove materials that are susceptible to SCC is corrosion testing. A variety of SCC corrosion tests exist for different metal alloy.
Examples[edit]
A classic example of SCC is season cracking of brass cartridge cases, a problem experienced by the British army in India in the early 19th century. It was initiated by ammonia from dung and horse manure decomposing at the higher temperatures of the spring and summer. There was substantial residual stress in the cartridge shells as a result of cold forming. The problem was solved by annealing the shells to ameliorate the stress.
A 32-inch diameter gas transmission pipeline, north of Natchitoches, Louisiana, belonging to the Tennessee Gas Pipeline exploded and burned from SCC on March 4, 1965, killing 17 people. At least 9 others were injured, and 7 homes 450 feet from the rupture were destroyed.[6][7]
SCC caused the catastrophic collapse of the Silver Bridge in December 1967, when an eyebarsuspension bridge across the Ohio river at Point Pleasant, West Virginia, suddenly failed. The main chain joint failed and the entire structure fell into the river, killing 46 people who were traveling in vehicles across the bridge. Rust in the eyebar joint had caused a stress corrosion crack, which went critical as a result of high bridge loading and low temperature. The failure was exacerbated by a high level of residual stress in the eyebar. The disaster led to a nationwide reappraisal of bridges.[8]
Suspended ceilings in indoor swimming pools are safety-relevant components. As was demonstrated by the collapses of the ceiling of the Uster (Switzerland) indoor swimming pool (1985), and again at Steenwijk (Netherlands, 2001), attention must be paid to selecting suitable materials and inspecting the state of such components. The reason for the failures was stress corrosion cracking of metal fastening components made of stainless steel. Further in 2004 a swimming pool in Moscow collapsed as caused by stress corrosion cracking [ref 1] resulting in 28 fatalities. The same for Chusovoy RU, resulting in 14 fatalities (2005, ref 1). And in 2011 a five month old baby got killed by stress corrosion cracking of the exotic stainless steel SS 1.4539 in Tilburg NL. Scientific research of NACE TG 498 confirmed that 1.4529 is very dangerous.[9] The active chemical was chlorine added to the water as a disinfectant.
See also[edit]
References[edit]
- Notes
- ^ASM International, Metals Handbook (Desk Edition) Chapter 32 (Failure Analysis), American Society for Metals
- ^Munnings, C.; Badwal, S. P. S.; Fini, D. (20 February 2014). 'Spontaneous stress-induced oxidation of Ce ions in Gd-doped ceria at room temperature'. Ionics. 20 (8): 1117–1126. doi:10.1007/s11581-014-1079-2.
- ^Wachtman, John B.; Cannon, W. Roger; Matthewson, M. John (11 September 2009). Mechanical Properties of Ceramics (2nd ed.). John Wiley and Sons. doi:10.1002/9780470451519. ISBN9780471735816.
- ^EPRI Search Results: Compressor Dependability: Laser Shock Peening Surface Treatment
- ^http://pbadupws.nrc.gov/docs/ML1116/ML11167A243.pdf
- ^http://primis.phmsa.dot.gov/comm/reports/enforce/documents/420101007H/420101007H_CAO_12032010.pdf
- ^The Washington Observer - Google News Archive Search
- ^Lewis, Peter Rhys, Reynolds, K, and Gagg, C, Forensic Materials Engineering: Case studies, CRC Press (2004).
- ^[1] RVS in zwembaden is als een kanarie in een kolenmijn. AluRVS 2017. [2] Province of Noord Brabant: 'Investigation on accident Reeshof Tilburg. 2012. [3] Proceedings of NACE TG 498. NACE International, Houston TX USA. 2015. M. Faller and P. Richner: Material selection of safety-relevant components in indoor swimming pools, Materials and Corrosion 54 (2003) S. 331 - 338.(only online in German (3.6 MB)) (ask for a copy of the English version)
- Sources
- ASM International, Metals Handbook (Desk Edition) Chapter 32 (Failure Analysis), American Society for Metals, (1997) pp 32–24 to 32-26
- ASM Handbook Volume 11 'Failure Analysis and Prevention' (2002) 'Stress-Corrosion Cracking' Revised by W.R. Warke, American Society of Metals. Pages 1738-1820
- 'Mechanical Properties of Ceramics' by John B. Wachtman, W. Roger Cannon, and M. John Matthewson. Chapter 8.

External links[edit]
- Decoupling stress and corrosion to predict metal failure: Arizona State University
Stress corrosion cracking (SCC) is difficult to predict and identify, and it can lead to catastrophic failure, often without any prior warning. Element’s SCC capabilities help to quickly evaluate the susceptibility of metallic materials to cracking and determine the size and scope of the problem before the failure occurs, avoiding major industrial costs and safety hazards.
SCC is the unexpected sudden failure of normal ductile materials when exposed to a combined effect of sustained tensile stresses and corrosion reactions. It can occur with both static externally applied loads and residual stresses from welding, forming, machining, grinding, physical damage, heat treatment, and operating stresses.
SCC often develops rapidly in a structure and can result in many significant threats such as external and internal corrosion, manufacturing defects, welding and fabrication defects, equipment failures and incorrect operations causing serious damage to the environment and a company’s reputation.
Combining industry experience with state of the art corrosion laboratory testing and simulation facilities, Element delivers a wide range of SCC testing in a variety of environmental conditions covering both, in-field inspection and laboratory analysis.
For more information about how we perform Stress Corrosion Cracking (SCC) Testing, or to request a quote, contact us today.
Our Stress Corrosion Cracking Testing Services
– Sour Service Cracking
- NACE MR0175 / ISO 15156
– Stress Corrosion Cracking
- ASTM G44, G47, G64 (aluminum)
- NACE TM0198, ASTM G129, ISO 7539-7 (SSRT)
- NACE TM0177, NACE TM0316
– Sulfide Stress Cracking
- NACE TM0177, NACE TM0316
- BS 8701
– Chloride Stress Cracking
- ASTM G36
– Ammonia Stress Cracking
- ASTM B858 (ISO 6957)
- ASTM G37
– Intergranular Stress Corrosion Cracking (IGSCC)
Materials We Test
Element’s corrosion testing laboratories offer a variety of SCC tests for a range of metals and alloys used in the Oil & Gas, Aerospace and Transportation industries.
– Carbon and alloys steels
– Stainless steels
– Nickel alloys
– Aluminum alloys
– Titanium alloys
– Copper alloys